How a new implant could treat pain without opioids
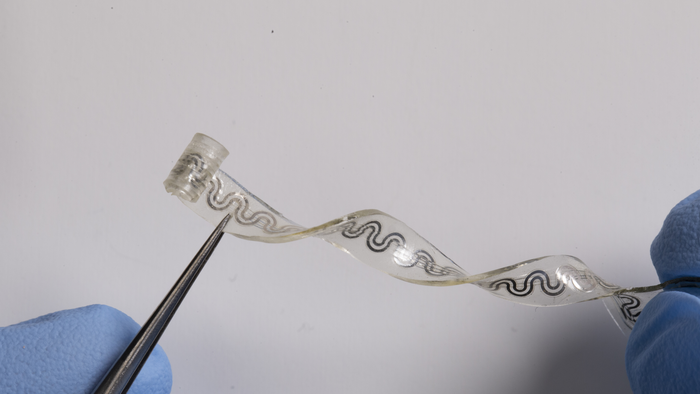
At some point or another, we all experience physical pain. We push through it or turn to medicine, from aspirin to opioids. New research published in the journal Science highlights a more mechanical alternative: a biodegradable implant that cools a nerve to block pain signals.
It looks like a small, clear rubber band with tiny channels snaking through it. Marketplace’s Kimberly Adams spoke with John Rogers, a biomedical engineer at Northwestern University who led the research and design of the device. He explained how the technology works and the challenges involved in managing chronic as well as acute pain. The following is an edited transcript of their conversation.
John Rogers: If you’ve ever been outside on a cold day without gloves on, your fingertips kind of become numb in many cases. And that’s a consequence of the cooling effect on propagation of signals through sensory nerves in the fingertips. We’re exploiting that same type of effect, but applied to peripheral nerves that are deep inside the body and are carrying pain signals from a particular region of the body up to the brain. By delivering that local cooling effect, we can block those signals. And we can do that in a reversible and a controllable manner that may provide in the future an alternative to drug or pharmaceutical-based approaches for managing pain.
Kimberly Adams: That’s such an important point. A lot of people get prescribed opioids for pain, and that has, in many ways, fueled opioid addiction. Is this a potential future solution to some of those problems?
Rogers: Well, we hope so. I’m a biomedical engineer, and we think about device- and technology-based approaches to addressing patient needs, ultimately. And pain management is an important part of patient care. And we became kind of aware of the opioid challenge and the addiction and all the adverse consequences of that sort of approach to managing pain and decided that maybe as engineers we could come up with a device-based alternative. And so this device is very targeted. It’s placed at a specific location in the body. It’s controlled in a deterministic manner, very much unlike drugs that kind of course through the entire body in an uncontrolled manner in many cases. And it also has a switch and a dial, so we can turn on and off the pain-relief effect. And we can adjust its magnitude. So quite a bit different than a drug-based approach, and we think it could offer some important advantages.
Adams: If you’re cooling the nerves enough to stop the pain signals, how do you avoid also stopping nerve signals that, say, make your muscles move?
Rogers: So it turns out there is a range of temperatures where you can block those pain signals without blocking the motor signals that are needed to control muscles in the body. So it turns out that if you have very tight control over the temperature, you can block pain without affecting motor behaviors.
Adams: Since you already knew that applying cold to a specific nerve could potentially numb or reduce pain, what is it about the distance between the surface of the skin and the location of the pain point that makes it hard to deliver that cooling to where you’re trying to go?
Rogers: Ultimately, the goal here is to deliver cooling power locally to a region of interest, but nowhere else. And so any kind of approach that doesn’t offer that capability will lead to cooling in unwanted locations of the body. And in certain instances, that cooling can turn out to be excessive in the sense that it can damage the adjacent tissues. And so our device and our ability to create this evaporative cooling effect only at the location of the peripheral nerve that we want to induce the pain block is a key feature of how we put the device together.
Adams: Opioids are often deployed to treat chronic pain. And it seems like what you’re talking about mostly here is more acute pain, like such as related to surgeries and things like that. Is there a deployment for this technology for chronic pain, like the type that is often treated with opioids?
Rogers: That’s definitely a goal of ours, is to use this development as maybe a starting point for treating chronic pain. We initially decided to focus on this acute postoperative pain opportunity because the device is an implantable system. And the way that it’s set up currently, it requires access to external hardware — pumping systems and access to these cooling liquids, for example — for it to operate properly. So I think this postoperative sort of hospital care and in an acute setting is a natural starting point based on certain features of the engineering. But that’s not to say that one couldn’t envision maybe a fully implantable version of this device, with a recirculating pump in miniaturized form, that can be inserted into the body, in which case you could easily envision it being used to treat chronic forms of pain. That will require some significant additional engineering development work, but it’s something that we’re very interested in.
Adams: How far away are we from maybe having this as a part of regular patient care, if at all?
Rogers: There’s some additional work that needs to happen around the biological effects; specifically, the time and the duration of this cooling that can be imposed on a nerve without any kind of irreversible adverse effect on the tissue itself. We need to do more of that kind of work and more studies of that type in larger-animal models. That’s one thing to bear in mind. The other is that a device of this sort represents a general class of technology that the [Food and Drug Administration] has not processed in the past. And so there are associated uncertainties that follow from that in terms of the regulatory process, but this is another kind of wild card in sort of predicting the time when this device would be available for widespread use with humans.

Related links: More insight from Kimberly Adams
Rogers said one benefit of this new device is that once you are done using it, it dissolves into the body over the course of about 50 days. Here’s a short video showing what that looks like.
While Rogers said the current device is best suited for acute pain, like from surgery, he said the implant may one day, with a few upgrades, be used to treat chronic pain and replace opioid treatments.
For now, there are other tech options for treating chronic pain. In November, the FDA approved an at-home virtual reality program that treats chronic lower-back pain.
While we’re on the topic of virtual reality, here’s an interview my colleague Meghan McCarty Carino did in April about how VR is being used not just to treat pain but to help diagnose certain ailments. So I guess the doctor will see you now — in your headset.
The future of this podcast starts with you.
Every day, the “Marketplace Tech” team demystifies the digital economy with stories that explore more than just Big Tech. We’re committed to covering topics that matter to you and the world around us, diving deep into how technology intersects with climate change, inequity, and disinformation.
As part of a nonprofit newsroom, we’re counting on listeners like you to keep this public service paywall-free and available to all.
Support “Marketplace Tech” in any amount today and become a partner in our mission.


















