FDA approves new Johnson & Johnson drug for depression

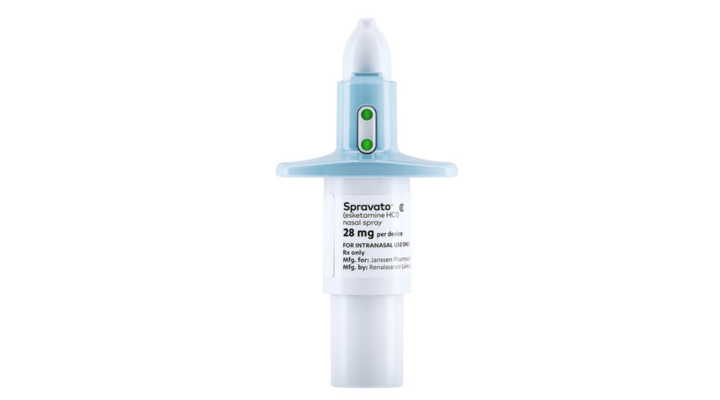
The Food and Drug Administration has approved the first new kind of drug to treat depression since Prozac hit the scene in the late 1980s. Spravato is a nasal spray from Johnson & Johnson that’s a close cousin of ketamine, an anesthetic that’s sometimes used recreationally and often known as “Special K.” Because ketamine can cause hallucinations and out-of-body experiences, the new drug must be administered in a clinical setting. The drug is designed to start working in hours instead of weeks. It’s meant to treat people who have not found relief through other antidepressants. That’s about 5 million of the 16 million Americans with depression.
Click the audio player above to hear the full story.
Correction (March 6, 2019): A previous version of this story misspelled Spravato. The text has been corrected.
There’s a lot happening in the world. Through it all, Marketplace is here for you.
You rely on Marketplace to break down the world’s events and tell you how it affects you in a fact-based, approachable way. We rely on your financial support to keep making that possible.
Your donation today powers the independent journalism that you rely on. For just $5/month, you can help sustain Marketplace so we can keep reporting on the things that matter to you.